We are proud to unveil our groundbreaking manufacturing solutions and latest technologies by offering additional new compliance driven capabilities in Drug Substance Manufacturing, Liquid Fill-Finish and Automated Visual Inspection. With these exciting investments and in combination with our process excellence, commercial expertise and an extensive array of in-house analytical capacities, we are able to provide our client’s products more flexible, quickly and on an even higher quality standard.
ASK US ABOUT OUR AVAILABLE CAPACITIYLatest Investments
With more than 100 years in vaccine manufacturing and more than 30 years in commercial fill-finish, we have a long-standing history in working with global pharma and biotech companies.
Supporting our clients in accelerating the development and flexible high-quality manufacturing of their products, we have once again significantly expanded our capacities.
IDT Biologika’s Latest Investments in New Capacities
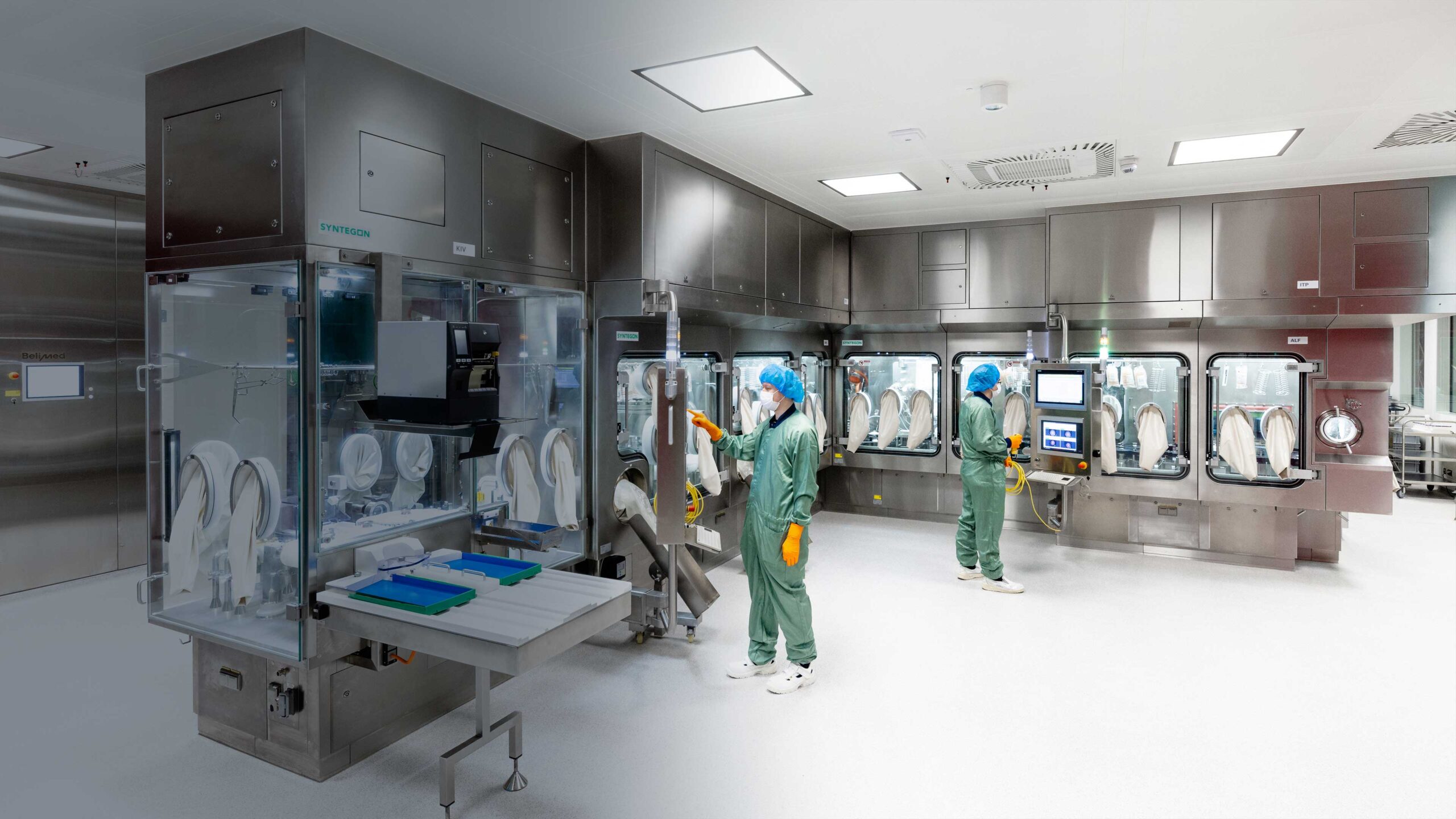
Drug Substance Manufacturing

IDT Biologika has set up an advanced new drug substance manufacturing facility.
The new facility houses production rooms with 2,000 L bioreactors for late-stage and commercial products as live viral vaccines, viral vectors for cell and gene therapeutics and oncolytic viruses. It ensures a great flexibility in upstream and downstream technologies.
Additional iCELLis® 500 are installed to manufacture adherent cells.
Fill/Finish

Aseptic filling in vials and pre-filled syringes as well as lyophilization of vials are core competencies at IDT Biologika.
For fill-finish of late stage and commercial products up to BSL-2 we have expanded our capacities for 2R, 6R and 10R vials (up to 50R possible) by a further dedicated state-of-the-art filling line.
With the capability to fill up to 80 to 100 million 2R vials per year and batch sizes of up to 500,000 2R vials, this line is one of the fastest in the world and is processing large and small molecules:
- Viral Vaccines
- mRNA and DNA Vaccines
- Proteins
- Virus-Like Particles
- Monoclonal Antibodies
- Oncolytic Viruses

Read this brochure and learn more about our new high-speed filling capacity.
Read BrochureVisual Inspection and Secondary Packaging

A high performance automated visual inspection line with a capacity of 36,000 2R vials per hour complement our capacities in manual and semi-automated visual inspection – especially for large batch sizes and commercial supply.
As contract manufacturer for the whole value chain of biologics production, the expansion of our packaging dimensions is a consequential step to the enlargement of our fill-finish capabilities. This includes labeling, blistering and packaging of our client’s healthcare products. We support integrated or standalone clinical and commercial packaging.