

About
IDT Biologika
IDT Biologika is a globally operating biopharmaceutical Contract Development and Manufacturing Organization that specializes in Vaccines, Cell & Gene Therapeutics and the Fill-Finish of Biologics and other Sterile Injectables. The company combines more than 100-years of expertise in research and production with state-of-the-art technology.
Our mission is to accelerate the development and flexible high-quality manufacturing of our client’s therapeutic and prophylactic products to treat serious diseases that impact human health worldwide.
Sites and Track Record
IDT Biologika´s site for clinical and commercial manufacturing is located in Germany. Our R&D Center, which specializes in process development and analytical services, is located in Maryland, USA.
Our excellent track record is manifested by successful inspections and approvals from major regulatory agencies including FDA, EMA and ANVISA.
Responding to Growth in Advanced Therapies and in Commercial Manufacturing
In response to the rapid growth and demand in Advanced Therapies, we also support the development and manufacturing of viral vectors for cell and gene therapeutics and are proud to be one of the first suppliers for commercial manufacturing of an oncolytic virus product, worldwide.
Our innovation, outstanding technological expertise and process excellence brings us to delivering for commercial programs for over three decades by providing liquid and lyophilized fill-finish of vaccines and other biologics, such as monoclonal antibodies and recombinant proteins. IDT Biologika has spent a century committed to the protection and improvement of human health worldwide, and our experienced international team is dedicated to continue this effort into the future.
Over 100 Years of Experience in Vaccines

In 2021, IDT Biologika reached the landmark of 100 years working in the development and manufacture of vaccines that protect humans all around the world. We continually put this unparalleled experience to work for our customers and in the last two decades have been involved in development and production of vaccines against infectious diseases such as COVID-19, influenza, smallpox, AIDS, malaria, Ebola, Chikungunya, Lassa fever, and Dengue fever. IDT Biologika has also worked on the production of vaccines against certain cancers. Our vision is to remain at the forefront of vaccines that offer critical protection to patient populations worldwide and continue to pioneer vital treatments for the next century.
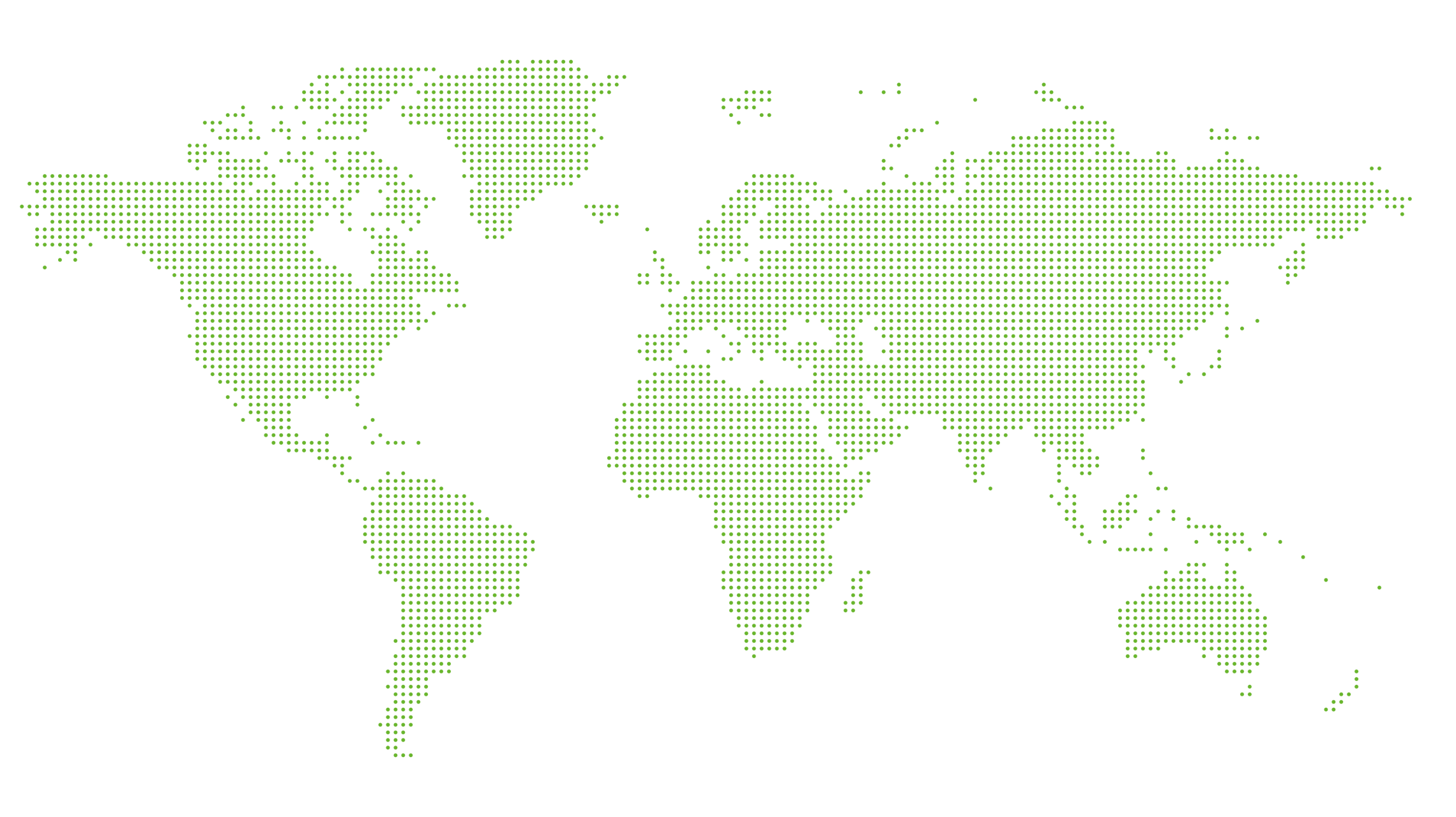
Fighting Infectious Diseases Worldwide
Occurence of infectious diseases and involvement of IDT Biologika (in Green).
For governmental businesses IDT Biologika has been providing emergency use vaccines for more than 15 years and we are also part of the German Government’s pandemic preparedness.
European & North American Footprint
IDT Biologika is a global biologics manufacturing organization, and we meet the needs of multinational customers and pharma companies around the world with manufacturing capabilities in Europe. We are headquartered in Dessau-Rosslau, Germany, with a second site in Magdeburg, Germany. Our North American facility is close to Washington, D.C., in Rockville, Maryland. These locations support our fully integrated services and are cGMP and BSL-2 -compliant, with approvals from the FDA, EMA, and ANVISA.
Strategic Locations
Rockville, Maryland, USA
Our US site has a long history in the production of biologics. Today, our R&D Center in Maryland specializes in the development of processes and analytical methods for vaccines and gene & immune therapeutics.
- Process Development
- Analytical Services
Full-Service CDMO Capabilities
At IDT Biologika, we recognize how critical vaccines and novel therapeutics are to protecting and improving quality of life for patients around the world. This is the inspiration for investment and growth of our business. We continue to expand our operations in Europe and North America, focused on delivering the highest quality end-to-end services with the ability to scale projects from development and preclinical volumes through to large-scale production and commercial packaging and supply. As part of a €114 million expansion, we are creating additional capacity in drug substance manufacturing, aseptic fill-finish, and visual inspection, which will be online and operational at the end of 2022. Everything we do is underscored by our commitment to quality and operational excellence.