Mathias Kahl, Stephan Bauer, Dr. Daniel Köhler, Dr. Tobias Thom, Rico Hinrichs, Stefan Borutzki, Christina Pospisil, Dr. Rico Schmidt, Erik Arnold, Dr. Thomas Kreisig
Excerpt
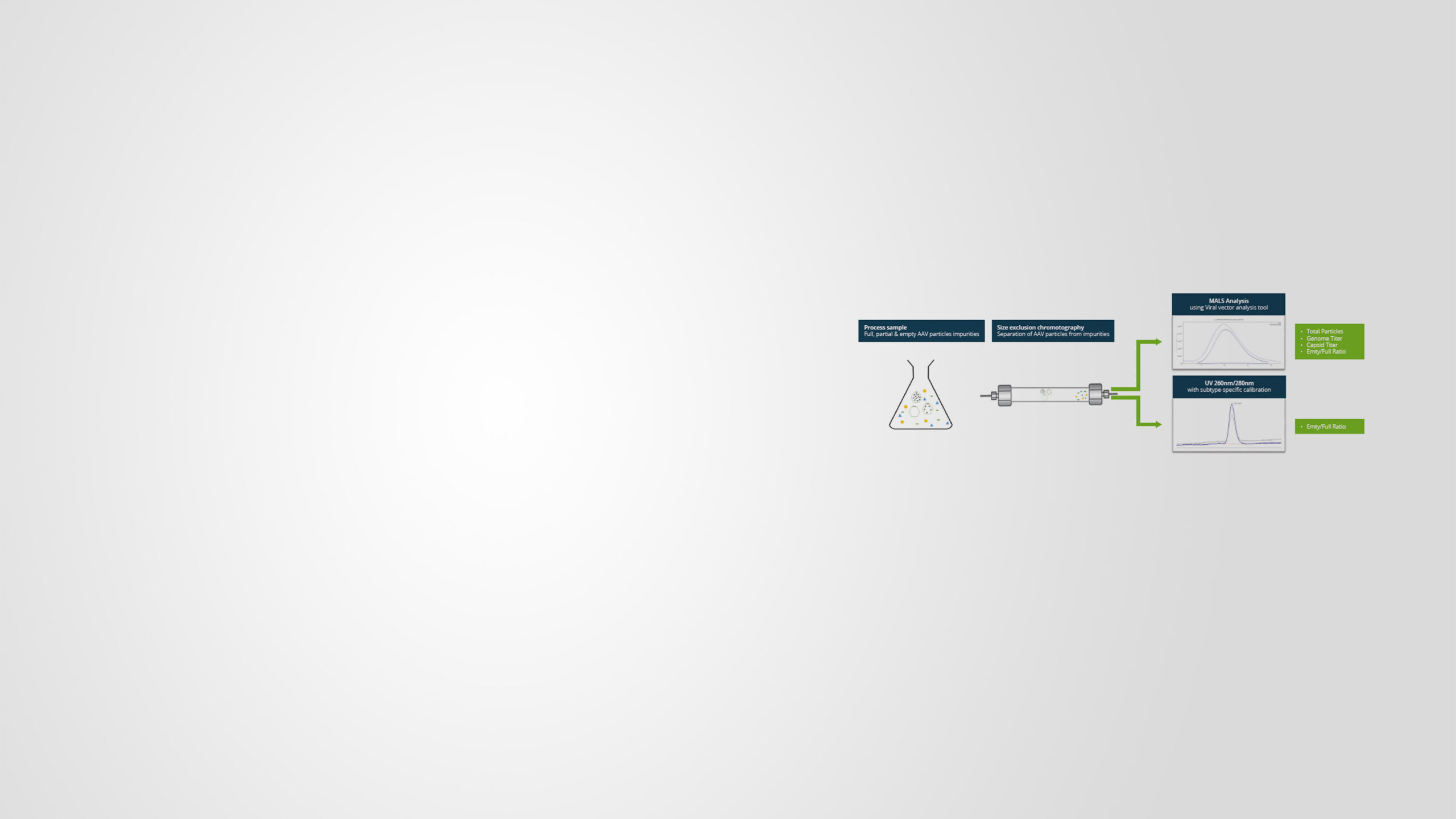
Cell and gene therapies are growing exponentially across the pharmaceutical field due to their potential to treat and possibly cure a wide range of devastating diseases, including cancer. As these therapies are becoming more wide spread, many drug sponsors are approaching trials with limited knowledge of how to design and launch a successful clinical trial manufacturing (CTM) process. This includes how to strategically investigate and manufacture adeno-associated virus vectors (AAVs) and lentiviral vectors (LVVs), two of the leading viral vectors used for gene therapeutics. Sponsors are faced with a stark learning curve that, without intervention, may lead to high costs, delays, and a failure to bring their drug to patients. …
Have a look at our Expert Content & Downloads section for more White Papers and Downloads from IDT Biologika.